|
This article is presented in two sections. Both are offered here. Section #1 is a simple overview of specific gravity and how to select then adjust for the best SG in the marine aquarium.
Section #2 offers more in depth information as to what specific gravity, salinity and density are. Section #1
Specific Gravity is the ratio of densities of ions in fresh water. These ions are different salts, minerals and/or elements. Some people mistakenly use the terms salinity or density. Different oceans have slightly different specific gravities. The tropical Indo-Pacific has an average specific gravity of 1.022 – 1.025. The Caribbean has an average specific gravity of 1.023 – 1.026. The Red Sea has an average specific gravity of 1.028 – 1.035. The specific gravity changes in each area are due largely to rain fall. All oceans are similar in ionic composition. The differences in water chemistry from ocean to ocean is a result of slightly different specific gravities. E.g. The higher the SG, the more ionic value. The home marine aquarium is a unique balance of nature and workable conditions that favor captive marine organisms. Some things in nature must be kept at similar levels in the marine aquarium. While other things should be slightly altered to better serve the captive animals on display. Phosphate, silicate and to a lesser degree specific gravity are things that should be at different values in the marine aquarium vs. nature. In natural seawater there is 0.07 ppm of phosphate. In the home aquarium, little to zero phosphate is desirable. Phosphate gradually accumulates as a waste ion in the closed re-circulating system. This can produce hair or slime algae.
In natural seawater there is 3.0 ppm of silicate. Silicates (dirt) should be found at near zero concentrations in the home marine aquarium. Silicates produce brown or diatom algae. Silicates in artificial seawater is usually introduced as impurities by the use of low grade raw materials employed in some economy marine salts. Some algae stimulants and/or reef additives used with a marine salt that is contaminated with silicate can result in red slime algae.marine aquarium. Silicates produce brown or diatom algae. Silicates in artificial seawater is usually introduced as impurities by the use of low grade raw materials employed in some economy marine salts. Some algae stimulants and/or reef additives used with a marine salt that is contaminated with silicate can result in red slime algae. High levels of phosphate or silicate foment the growth of undesirable algae. Most marine fish do better in an aquarium with slightly lower specific gravity vs. their natural habitat. Reef invertebrates from the same ocean do better at a specific gravity similar to their natural habitat. If marine fish come from an environment which has a SG of 1.024, they will do better in an aquarium with a SG of 1.021 – 1.022. This basic guideline of lowering SG about 0.002 – 0.003 vs. natural habitat generally can be used for most marine fish. There is more dissolved oxygen in lower specific gravity water. Even 0.002 to 0.003 can make a difference. Lower SG’s than 0.009 can result in the loss of fish color, lack of vigor and general overall health decline. When treating marine fish for parasite related disease, it is always best to lower SG about 0.004 – 0.005 less than their natural habitat. This is done to slow the multiplication of parasites. Lower SG will allow the proper medication to control and ultimately eradicate itch, velvet, etc. Adjusting Specific Gravity. For marine aquarium use, any hydrometer that is used must be calibrated to 1.000 G/cm3, in the purest water possible, at a specific temperature. E.G. Float a glass hydrometer in clean fresh water at 77°F. It should read 1.000. If not, then readings in salt water, at the same temperature must be compensated for. To lower specific gravity, simply add more clean fresh water to your salt solution. To raise SG, simply add more marine salts. Remember, the quality of your salt water is directly related to the quality of the fresh water you use to mix salts. If you suspect your fresh water supply to be contaminated with heavy metals, phosphates, insecticides, pollutants, it is better to use purified water. Many aquarists choose a Reverse Osmosis filter. The RO filter is used to filter tap water. It is not intended for use as an aquarium filter. The RO filter removes about 90%+ of all undesirables listed above. It also makes great drinking water. For marine aquariums there are two popular types of hydrometers. Floating glass hydrometers and molded plastic forms with indicator arms. There are two classifications of floating glass hydrometers.
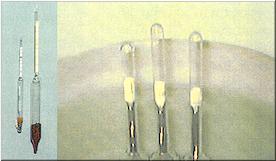
The low cost floating glass hydrometers are usually 6 1/2″ to 8 1/2″ tall. Most are equipped with a thermometer. Very few meet the requirement of floating at 1.000 in clean fresh water. Initial errors must be compensated for when attempting to use them. These are usually produced in: Korea, Taiwan or Mainland China. This type usually retails for $2.99 to $8.99. These “economy” hydrometers are generally agreed to be useless.
The high cost floating glass hydrometers are usually 10″ to 13″ tall. Some are equipped with a thermometer. Most meet the requirement of floating at 1.000 in clean fresh water. These are usually produced in: Japan, USA or Germany. These better glass hydrometers usually retail for $15.99 to $34.99. Laboratory glass hydrometers usually retail for $49.99 to $79.99. Both types of glass floating hydrometers share the same limitations. They can easily break. There are lead beads at the bottom of glass hydrometers. If broken in the marine aquarium, the lead will ruin the tank water and broken glass cannot be seen in water. There are two classifications of plastic hydrometers. 1) Ones that work. 2) Ones that do not work.
Plastic hydrometers are a great idea. However, to produce them so they offer consistently accurate results from hydrometer to hydrometer is easier said than done. Plastic hydrometers consist of three pieces. 1) The form (box or tube). 2) The indicator arm, which points to numbers. 3) A special counter weight that goes inside of the indicator arm. One would think that each molded piece of plastic would be the same. This is basically true with the form and indicator arm. However, the special counter weight that is located inside the indicator arm is the key component. The slightest difference in weight has the greatest impact on accuracy. Section #2 Specific Gravity, Salinity, Density Hydrometers that show both the specific gravity and salinity are confusing and considered useless. Hydrometers that show density are incorrect and meaningless. The density of seawater is a function of the salinity, temperature and pressure. For obvious reasons, precise density determinations of seawater are extremely difficult to perform, and in use of the term in context with artificial seawater is misapplied. The term “salinity” is meaningless when used in reference to artificial seawaters. Salinity identified as total weight of solutes in 1kg of seawater. From the start this simple definition presented an operational difficulty: the obvious analytical technique of evaporating a sample of seawater to dryness results in loss of some volatile constituents, particularly hydrogen chloride. To compensate, the classic definition, formulated at the beginning of the 20th century, states that salinity is the total mass in grams of all dissolved substances per kilogram of seawater, with all carbonate converted to oxide, all bromide and iodide replaced by chlorine and all organic matter oxidized to 480°C. The density of a substance is the mass per unit of volume, commonly expressed as g/cm3. Specific Gravity is the ratio of densities, in this case the ratio of the density of seawater to the density of pure water at 4°C, at which temperature is presumed to be 1.000 g/cm3. Section 2 offers information found in ARTIFICIAL SEAWATER Formulas and Methods. Pages 2 & 3. Authors Joseph P. Bidwell and Stephen Spotte. Printed by Jones and Bartlett. ISBN 0-86720-057-X. |